Organophosphorus Chemistry
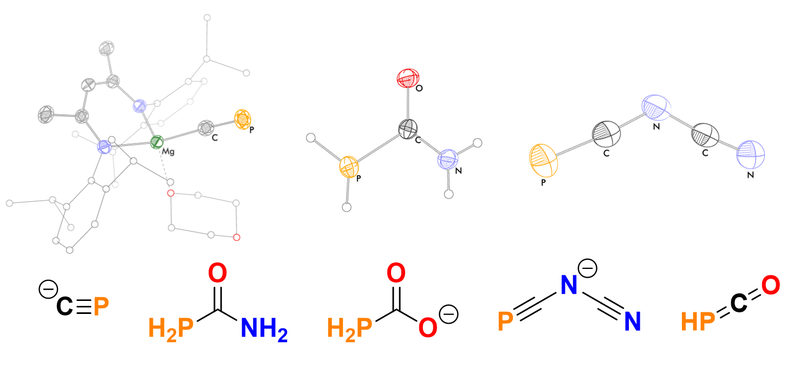
Phosphorus is often referred to as the “carbon copy” because it is isolobal to many common fragments in organic chemistry – including unsaturated, conjugated motifs. Our research focuses on developing the synthesis of novel organophosphorus compounds, and investigating their properties and reactivity.
We have synthesized phosphorus-containing analogues of many simple organic molecules, including cyanide, urea, and isocyanic acid. These organophosphorus compounds provide entry points for the synthesis of more complex phosphorus-containing molecules, and have potential applications for use in other fields such as materials science.
Recently, we have found that a magnesium complex of the cyaphide ion (C≡P–) can be prepared by the reduction of a silyl-functionalized phosphaethynolate. By analogy to Grignard reagents, this can be used to transfer the cyaphide ligand into the coordination sphere of metals through straightforward salt-metathesis reactions.[1]
PCO– can also be derivatised by reaction with amines under acidic conditions, yielding the corresponding phosphinecarboxamides. These inorganic analogues of urea are rare examples of air-stable primary phosphines, with promising materials applications.[2]
[1] D. W. N. Wilson, S. J. Urwin, E. S. Yang, J. M. Goicoechea, J. Am. Chem. Soc. 2021, 143, 10367–10373.
[2] A. R. Jupp, J. M. Goicoechea, J. Am. Chem. Soc. 2013, 135, 19131–19134
Heavy Cyanate Analogues
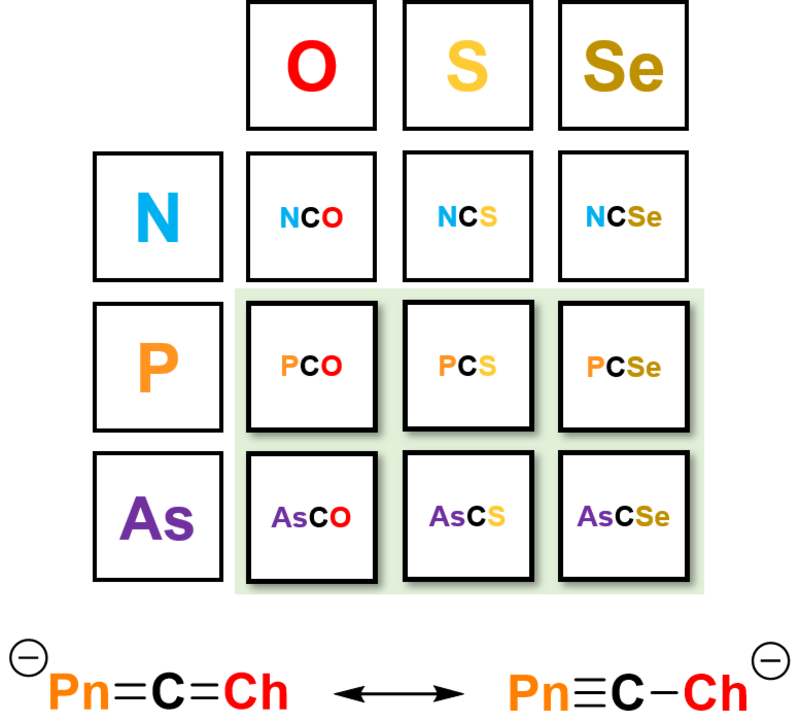
The phosphaethynolate anion, PCO–, is a heavy analogue of the valence isoelectronic cyanate ion (OCN−). The phosphaethynolate anion has opened an exciting new avenue of research in the field of organophosphorus chemistry; development over the past 8 years has demonstrated its versatility as a chemical precursor towards, for example, the synthesis of phosphorus-containing heterocycles, as a monoanionic phosphide source, and for low-valent compounds.[1b][2b]
The success of the 2-phosphaethynolate ion prompted us to explore the chemistry of its heavier analogues. The arsenic‐containing ion, AsCO−, was first isolated in 2016 by our research group, and promises to be a similarly versatile reagent in chemical synthesis, having already been employed for the synthesis of several novel molecules.[3b]
We have expanded the series further through a modular approach to the modification of the synthesis. This approach gives access to substitution at both the pnictogen and chalcogen sites, yielding a family of heavy cyanate analogues for further exploration.[4b]
[1b] J. M. Goicoechea, H. Grutzmacher, Angew. Chem. Int. Ed. 2018, 57, 16968-16994
[2b] A. R. Jupp J. M. Goicoechea, Angew. Chem. Int. Ed. 2013, 52, 10064-10067
[3b] A. Hinz, J. M. Goicoechea, Angew. Chem. Int. Ed. 2016, 55, 8536-8541
[4b] F. Tambornino, A. Hinz, R. Koppe, J. M. Goicoechea, Angew. Chem. Int. Ed. 2018, 57, 8230-8234
Metal and Metalloid Clusters
Our research group is interested in the solution-phase reactivity of “naked” polyanionic clusters of the group 14 and 15 elements.[1c,2c] These species can be used as precursors for a wide range of compounds with interesting structures, many of which disobey established rules for bonding. For example, we have pioneered the study of open-shell main group clusters featuring interstitial transition metal atoms, such as [Fe@Ge10]2− and [Ru@Ge12]3− (Figure 1).[3c,4c] These species represent molecular models of transition-metal/main-group alloys which have found numerous applications in materials science and catalysis.
More recently, we have focused further into the applications of Zintl clusters towards catalysis. For example, the reaction between [Rh(COD)Cl]2 and K[Ge9{Si(SiMe3)3}3] produces a neutral, alkane soluble cluster, [η4-Ge9{Si(SiMe3)3}3]Rh(COD) (Figure 2), which is the first example of a Zintl cluster homogeneous catalyst, catalysing the hydrogenation or cyclic alkenes at room temperature.[5c]
These promising results represent the first steps in a nascent area of chemistry where many interesting breakthroughs await.
[1c] S. C. Sevov, J. M. Goicoechea, Organometallics 2006, 25, 5678.
[2c] R. S. P. Turbervill, J. M. Goicoechea, Chem. Rev. 2014, 114, 10807.
[3c] B. Zhou, M. S. Denning, D. L. Kays, J. M. Goicoechea, J. Am. Chem. Soc. 2009, 131, 2802.
[4c] G. Espinoza-Quintero, J. C. A. Duckworth, W. K. Myers, J. E. McGrady, J. M. Goicoechea, J. Am. Chem. Soc. 2014, 136, 1210.
[5c] O. P. E. Townrow, C. Chung, S. A. Mcgregor, A. S. Weller, J. M. Goicoechea, J. Am. Chem. Soc. 2020, 142, 18330.


 The College of Arts
The College of Arts